Latest articles
- Progres® Liquid: Advancing disease resistance and growth performance in Asian Seabass – Aquaculture Europe Conference 2023
- Effects of Progres® on white shrimp – Aquaculture Europe 2023 conference
- Progres® studies to be presented at Aquaculture Europe 2023 conference
- Hankkija has sold the Progres® feed innovation business to AB Vista
- Progres® and Progut® in the weaner diet increased piglet resilience to F4-ETEC in the post-weaning period – A poster from Schothorst Feed Research at the ZeroZincSummit2022
- Raising piglets without medical ZnO – an example from Finland
- Key Insights from the IHSIG 2024, Manila Philippines
- R&D network and feed innovations
- Effect of Progut®Extra on horse immunity, gut microflora and well-being

The role of Progut® in preventing pathogen adhesion in the nutrition of monogastric animals
In feeding of monogastric animals, yeast products are commonly used to enhance performance by reducing the growth of pathogenic bacteria and promoting immunity. However, the efficacy of yeast products might vary a lot because of different raw materials and production technologies. The immense number of yeast products available makes it hard for farmers and nutritionists to distinguish between different yeast products. To evaluate yeast products, it’s essential to understand the factors that contribute to their effects.
How pathogens mistake yeast particles for the intestinal mucus
Biological functions between host animals and microbes are based on molecular interactions. Size, shape, electrical charges and other physical properties of active molecules are of key importance for these interactions.
Intestinal pathogens like E. coli and Salmonella colonize the gut by attaching to the intestinal mucus via filamentous structures called fimbriae. The attachment is mediated by the matching of the physical structures of the fimbriae with the carbohydrate structures of the mucus, like a key fitting a lock. The mucus contains mannose-rich glycoproteins which serve as attachment sites for mannose-sensitive fimbriae of some pathogens. However, only the three-dimensional structure of the glycoprotein, not just the presence of mannose, determines attachment.
Yeast cell walls contain similar carbohydrate structures, like mannose-rich glycoproteins, that can serve as attachment sites for pathogens. When yeast products are used, pathogenic microbes might attach to specific parts of yeast cell walls instead of the intestinal mucus. This prevents pathogens from colonizing the gut lining. This explains why the treatment of the yeast is crucial for the efficacy of resulting products. It’s the three-dimensional structure of the yeast cell wall compounds, which is needed for fimbriae attachment, not just its chemical composition. So, to create an efficient yeast product, we need a process to increase the amount of these active yeast cell wall structures.
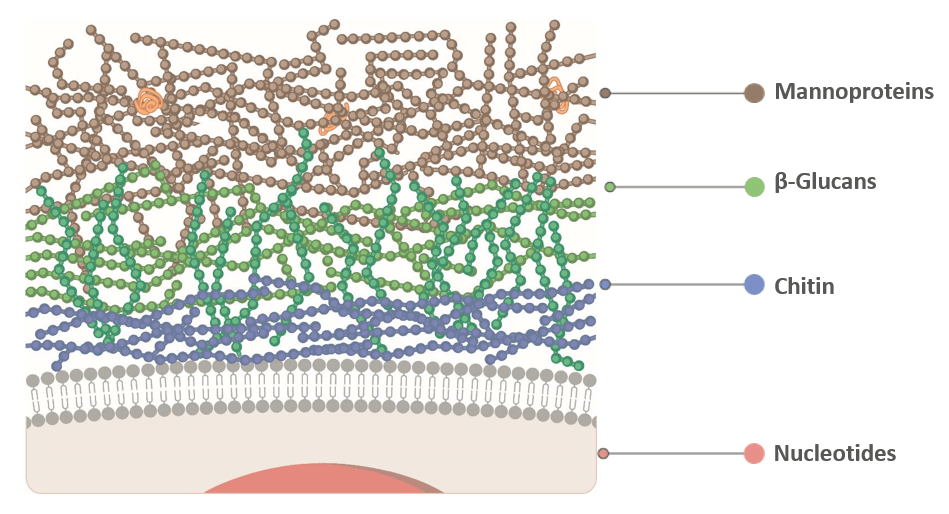
Controlled hydrolysis unlocks the potential of yeast cell wall components
The yeast cell wall is made up of mannoprotein, beta-glucan, and chitin (Figure 1), arranged in a complex structure. In its intact form (live yeast, inactivated feed yeast, yeast culture products), the structures are tightly bound and not that accessible to pathogenic fimbriae. The cell wall evolved to protect the cells, not to bind pathogens, indeed. To maximize the efficacy of pathogen attachment, we open the yeast cell wall using a controlled and optimized hydrolysis method (Figure 2).

A precisely adjusted hydrolysis (by acid or enzymes) can split yeast cells into right structures, thus increasing the efficacy of resulting particles in binding pathogens. Thanks to hydrolysis of yeast cells, in the extract emerge fractions rich in water-soluble oligosaccharides. Those oligosaccharides (size: 5–20 sugar units) have more effective binding sites for bacterial fimbriae than pure mannose or long polysaccharide chains.

Figure 3. All yeast products offer binding sites for pathogenic bacteria and immune cells. The optimized hydrolysis of Progut® results in an increased water-solubility and thus multiplied availability of binding sites, compared with competing yeast products.
But in production of well-known MOS products this extract is separated. As a result, MOS products have a higher content of mannose and beta-glucan, but a lower content of active oligosaccharides. But both pure mannose and long, insoluble polysaccharide chains provide fewer binding sites for bacterial fimbriae than oligosaccharides: the ends of the chains are the only attachment sites! The whole yeast hydrolysate product, Progut®, contains both the extract fraction (rich in soluble oligosaccharides) and the cell wall fraction. This, combined with optimized hydrolysis, results in a product with a high number of suitable binding structures for E. coli and Salmonella (Figure 3). In numerous trials, our whole yeast hydrolysate has been shown to effectively prevent the adherence of E. coli and Salmonellato intestinal mucus compared to other live yeast, cell wall, and yeast culture products.






